Policy Papers
ON360 Transition Briefings 2022 – An Innovation Pathway to Turn Cancer Discoveries into Transformative Care
In Ontario, innovations in cancer research are not easily adopted into cancer care. A gap exists in the transfer of knowledge among research, health policy and patient care. Without better alignment to bridge the gap, Ontarians are missing out.

Issue
Ontario is home to world-leading scientists developing new insights into how to prevent, detect, diagnose and treat cancer. It also boasts one of the world’s finest publicly funded healthcare systems, with expert clinicians providing evidence-based clinical care.
Yet innovations in cancer research are not easily adopted into cancer care in Ontario. A gap exists in the transfer of knowledge among research, health policy and patient care. Without better alignment to bridge the gap, Ontarians are missing out.
A century of research has revolutionized our understanding of cancer. We know now that cancer is not one but hundreds of different diseases that develop uniquely in each patient and require individualized treatment strategies. The future of ‘personalized medicine’ is here, but new knowledge, discoveries and innovations need to reach the clinic more quickly and more often so that Ontarians can benefit fully. We also need to harness every patient experience as an opportunity to learn and improve.
We can bridge this gap by adopting a “cancer innovation pathway”: a formal structure to appraise and evaluate new technologies and processes in cancer care, implementing those that will benefit patients and steering clear of those that won’t. Aspects of this pathway will be new, while some of it will leverage structures that already exist in Ontario.
By creating a nimble, structured pathway to translate research into results, the province can build not just one of the most equitable cancer care systems in the world, but also one of the most innovative, where every patient can benefit from outstanding homegrown and global research.
The Need for Reform: Stakeholder Perspectives
Conversations with different stakeholders reveal how they currently interact with cancer research, and the challenges that must be overcome to harness it effectively.
- Patients want access to the newest innovations in cancer care that deliver the best health outcomes with the fewest side effects. But they must often travel out of province or out of country to access innovations that aren’t yet available in Ontario, even paying out of pocket for potentially life-saving therapies. Remote, rural and minority populations have less access to genetic tests, for example. This leads to sociodemographic inequities in quality of care and health outcomes.
- Governments can come under pressure to adopt new technologies and processes that have not been consistently evaluated because of a lack of evidence standardization. That makes it difficult to know which innovations will make an impact and how to implement them safely, efficiently and cost-effectively.
- Clinicians want to use new technologies to help their patients, but find they are bogged down by regulatory restrictions and slow accreditation processes. They and the hospitals where they work also face challenges learning to interpret new tests and implement new procedures.
- Clinical lab specialists can face pressure to conduct tests that are expensive, not yet standardized and not fully reimbursed to guide treatment strategies for their patients.
- Researchers spend years generating evidence to support their discoveries and express frustration at not knowing how to overcome the regulatory hurdles required to bring their innovations into clinical care in Ontario.
- Industry is discouraged from investing in Ontario cancer innovations by long and uncertain timelines for approval and reimbursement.
The Need for Reform: Why Now?
Ontario’s healthcare system is under pressure like never before, and the situation may get worse before it gets better. The province is aging, with seniors expected to comprise about a quarter of the population by the 2040s.
At the same time, the pandemic is pushing the health system to its limits and taking an especially hard toll on people with cancer. They are often at higher risk of illness and death from COVID-19 due to compromised immune systems and comorbidities, and many have had cancer treatments delayed because of pandemic restrictions. Cancer screenings are also down since March 2020 and experts are warning of a wave of late-stage cancer diagnoses that will further strain the system and cause unnecessary deaths.
Meanwhile, years of foundational scientific discoveries and data from innovative population health studies and clinical trials have fueled dramatic advancements in early detection, diagnosis and therapeutics. New minimally-invasive blood and urine tests, for example, can identify cancer earlier when it is most treatable, keeping patients healthier, keeping hospital beds free, and keeping costs down. Biological signatures of disease called biomarkers can be used to determine what is driving tumour growth and therefore what precise treatment will be most effective for a given patient. New ways of harnessing a patient’s own immune system can be used to target and kill cancer cells.
These Ontario-led innovations are already reshaping how we identify and treat cancer. But to benefit Ontarians, they must be integrated into clinical care more often and more quickly. This will require a clearer and more agile evaluation and approval process for novel tests and treatments. That pathway currently does not exist. The Ontario government has long recognized and supported the province’s cancer research community. But Ontarians will not benefit from the full impact of their investments without more effective means to determine which innovations work best.
How to Move Forward: A Cancer Innovation Pathway
Ontario needs a structured, transparent and multi-staged pathway to move discoveries out of the lab and into the clinic that leverages existing mechanisms for assessing innovations and builds new ones to ensure they are effective.
The pathway will need to be nimble to allow for iterative technological development and rapid progress, and broadly applied to cancer innovations. It will need a bias toward permissiveness so that more innovations are trialed in controlled settings and it must be backed by streamlined decision-making that readily discontinues innovations that don’t prove effective.
Decision-making structures must be transparent to build trust among diverse stakeholders and to leverage existing systems and organizations. The pathway must also enable a feedback loop of data sharing among researchers, clinicians and patients to foster a learning health system in which each patient’s experience informs better care for the next.
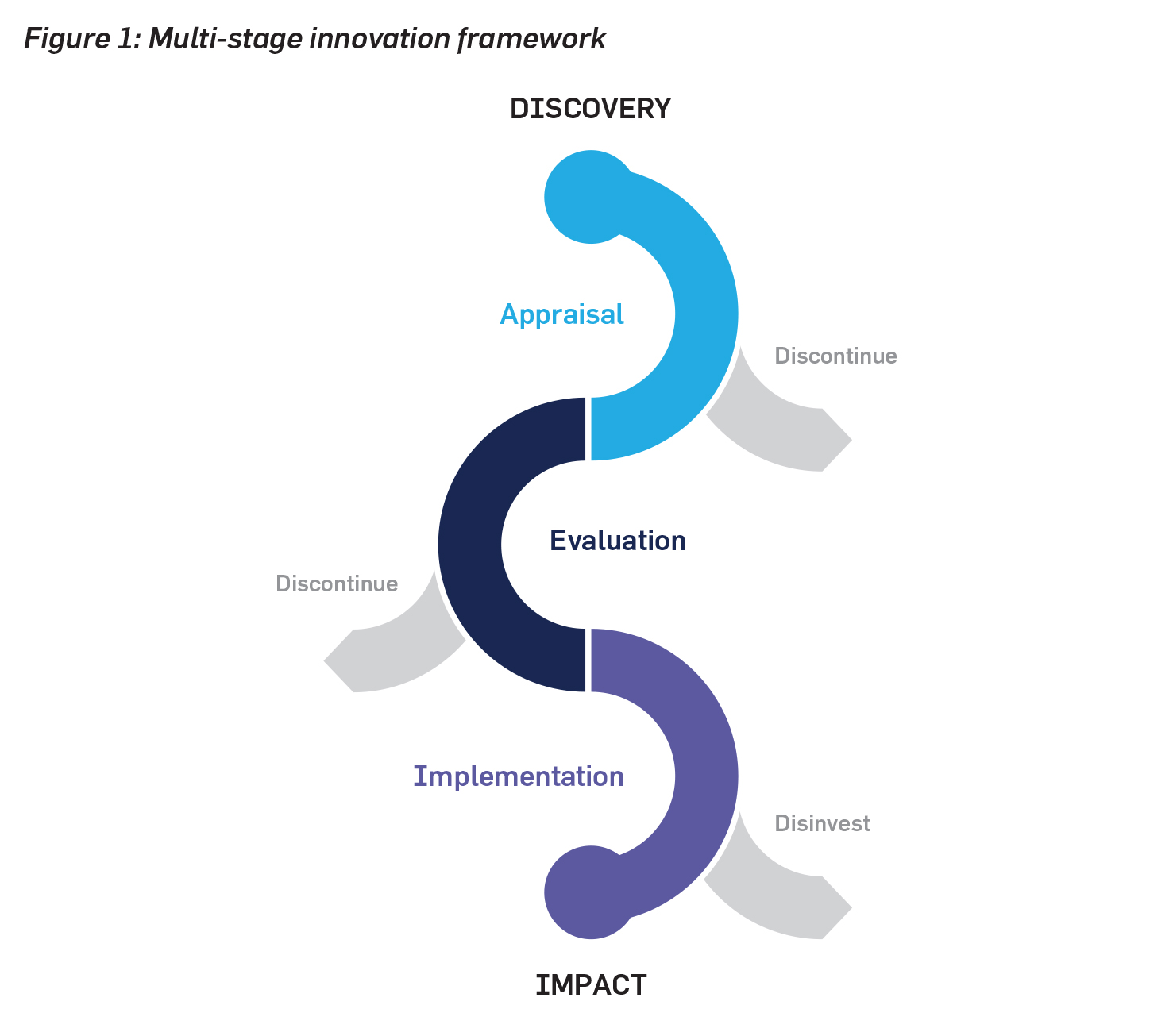
An innovation pathway framework to bridge between research discoveries and clinical impact would consist of three phases: (1) appraisal, (2) evaluation, and (3) implementation.
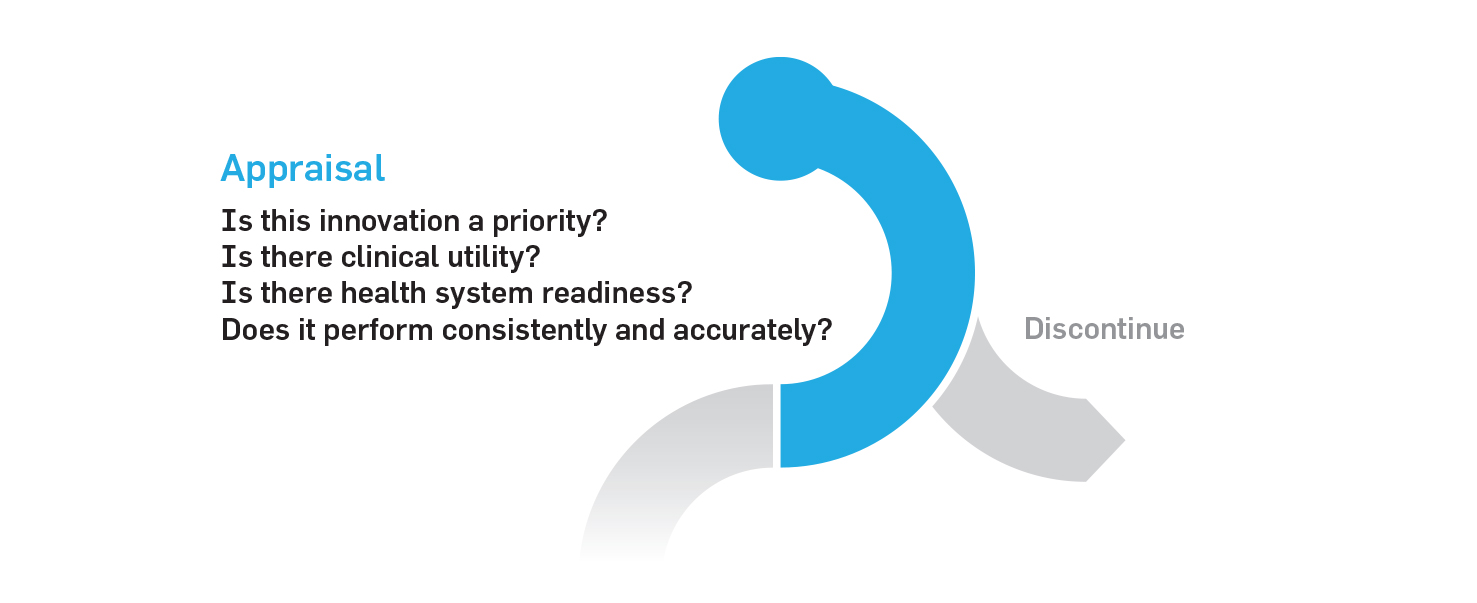
1. Appraisal phase
The appraisal phase provides a clear entry point into the pathway for new discoveries. At this step, innovations can be quickly assessed for clinical utility and prioritized for evaluation. Ones deemed successful at the appraisal phase would continue to the evaluation phase.
The costs of appraising such innovations could be covered by private-public partnerships which have been successful in developing hospital infrastructure in Canada. The academic or industry sponsors behind each discovery would be responsible for generating the evidence of clinical utility.
Several organizations already perform this type of work and could be leveraged as part of this pathway, including Ontario Health and the Canadian Agency for Drugs and Technologies in Health (CADTH).
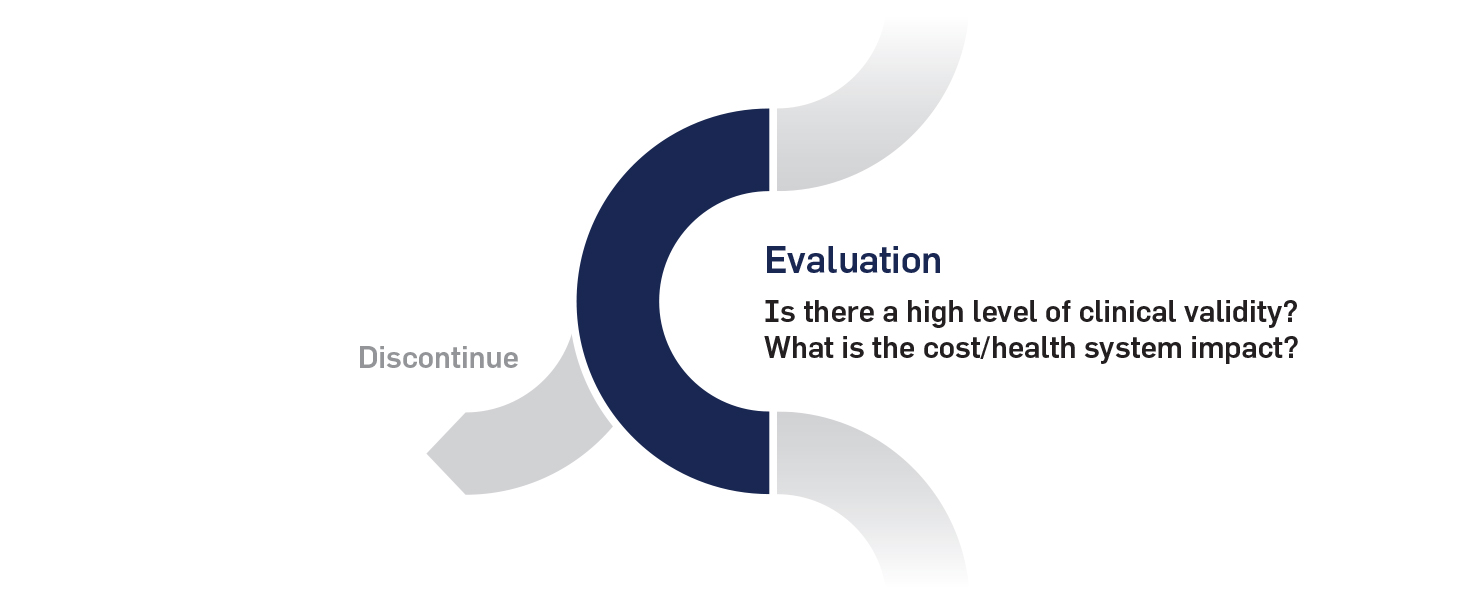
2. Evaluation phase
The evaluation phase is the critical evidence-based step to determine whether an innovation should be widely implemented. An innovation that is successful at the evaluation phase would be advanced to the implementation phase.
The appropriate levels and sources of evidence (i.e., randomized control trials, cohort studies, etc.) would need to be determined for different categories of innovation and different disease needs. Evidence development frameworks would be conducted with existing evaluation organizations. Instead of starting from scratch, the evaluation phase could be added to the mandate of one or several other existing evaluation organizations in the province or even at the national level, with financial support from private-public partnerships.
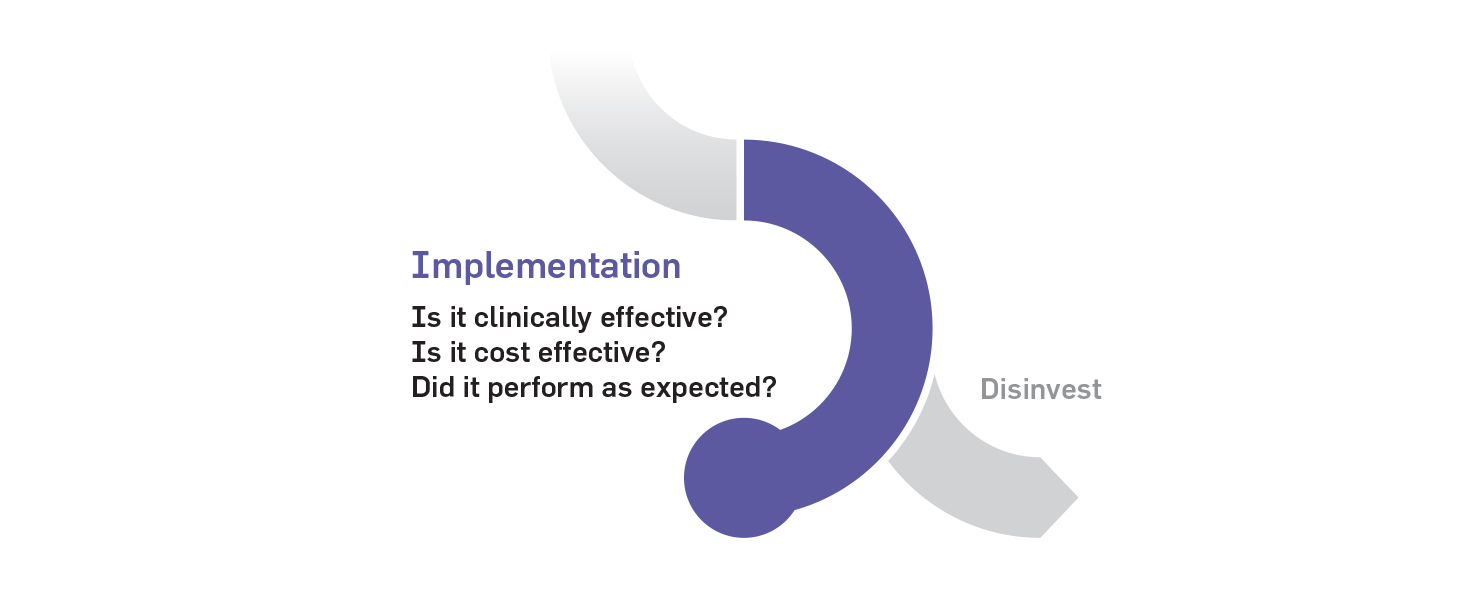
3. Implementation phase
The implementation phase is the stage at which innovations are adopted into clinical practice using a “scale and spread” model, in which successful pilot studies are quickly expanded and replicated across the province as best practice. An innovation pathway oversight committee will assess how new technologies and processes are performing in a real-world setting and determine whether they should continue to be widely adopted or discontinued.
As with other stages in the pathway, public-private partnerships could play a key role in supporting the costs and infrastructure required for implementation. The responsibility for scaling clinical implementation of innovations would rest with Ontario Health as the provincial health agency. The collection of real-world data to support effectiveness analyses and health system research could be supported through research grant funding and/or through quality and performance assessments conducted by Ontario Health or the Institute for Clinical Evaluative Sciences (ICES). New provincial investments in data sharing infrastructure, such as the Ontario Health Data Platform, would be critical to facilitate data sharing between research and clinical communities.
How to Move Forward: Next Steps
The challenges described above are well acknowledged across the spectrum of communities in the cancer research and care systems. This recognition should speed the path to an agreed-upon solution.
It will be critical to determine who owns the innovation pathway to ensure clear lines of accountability. One logical solution would be for the pathway to be governed by Ontario Health under delegated authority and with the support of the Ministry of Health. An innovation pathway oversight committee consisting of stakeholders from the research, policy, healthcare and patient communities would help to identify important innovations and move them efficiently through the pathway. The oversight committee could be linked to existing provincial committees such as the provincial genetics advisory committee (PGAC) or the clinical leads engaged with Cancer Care Ontario and Ontario Health to access specialized technical expertise when needed.
Determining the pathway’s scope among the many areas of cancer innovation will help set priorities and achievable goals.
Collaborations with existing health technology assessment bodies and national agencies also will be key to establishing evidence frameworks, data checklists and to creating transparent structures for evaluation.
How to Move Forward: Who Benefits?
Building a structured pathway from the lab to the clinic would benefit all stakeholders by bringing new innovations to clinicians and the patients they care for, and by making research more impactful and responsive to the needs of Ontarians.
- Patients will benefit from more cutting-edge innovations, and see their needs, perspectives and improved outcomes reflected in the care they receive.
- Researchers will enjoy better collaboration across all elements of the research system, gaining support to generate evidence in a timely fashion and seeing their knowledge, discoveries and innovations more rapidly translated into clinical care.
- Clinicians will be empowered to harness new technologies and techniques, working in a culture of innovation and constant improvement, knowing the care they provide is supported by evidence.
- Industry will be incentivized to invest in research and development in Ontario and to contribute their innovations to a province committed to collaboration, data-sharing and a rapidly learning health system.
Conclusion
The dedicated professionals in Ontario’s cancer research and cancer care sectors share the same goal: better health outcomes for people with cancer. But without a clearly marked bridge between their two sectors, they won’t have the fullest range of tools by their side.
A structured, transparent innovation pathway will turn more research innovations in Ontario into cancer care for Ontarians. It will foster a more effective healthcare system that emphasizes value, supports integrated data sharing practices and is anchored in patient needs. By embracing more innovation, including by learning from every patient’s clinical experience, Ontario’s cancer system can become the envy of the world.
Dr. Christine Williams is Executive Vice President and Head of Implementation Science at the Ontario Institute for Cancer Research.
Funding for the Ontario Institute for Cancer Research (OICR) is provided by the Government of Ontario. The views expressed in this paper are the views of OICR and do not necessarily reflect those of the Province.